Chemical Treatments
Chemical Safety Data
|
|
|
|---|
Thymol Safety Data
Safety information from various sources regarding thymol crystals that are use for beekeeping purposes.
|
Chemical Treatments Chemical Safety Data |
Thymol Safety DataSafety information from various sources regarding thymol crystals that are use for beekeeping purposes. |
|---|
|
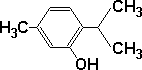
There is some confusion in the application of the words 'thymol' and 'thyme oil'. I use the word 'thymol' in this document to mean a common phenol that exists as a white crystalline solid at room temperature. Formula: C10H14O and molecular structure as indicated in the diagram at right. |
|
|---|
Thyme oils come from several different plants, some of which contain more than one type of essential oil.
- Trachyspermum ammi (Ajowan)
- Thymus (chemotype thymol)
- Thymus (chemotype carvacrol)
- Thymus serpyllum (Wild thyme or mother of thyme)
- Thymus vulgaris (chemotype thymol)
- Thymus vulgaris (chemotype linalol)
Thymol is only very slightly soluble in water, but has a strong affinity for fats and oils (Lipids). The degree of this solubility is represented by the following Compound Partition Coefficients (P or p):-
| Substance | Coeff. (P or p) | Reality |
|---|---|---|
| Ethanol | 0.1 | Higher solubility in water than fat |
| n-BuOH | 0.65 | Slightly more soluble in water than fat |
| Acetylsalicic Acid | 1.13 | Only a tiny bit more soluble in fat than in water |
| Benzamide - PhC(O)NH2 | 2.50 | Higher solubility in fat |
| Thymol | 950 | Very high solubility in fat |
Other names by which Thymol is known:-
- 2 isopropyl 5 methylphenol
- mint flavouring
- 6 isopropyl m cresol
- 3 hydroxy p cymene
- isopropyl cresol
- 4 methyl 2 hydroxy isopropyl benzene
CAS No:- 89-83-8
EC No:- 201-944-8
Appearance:- White crystalline solid or powder with a pungent odour.
Melting point = 49°C
Boiling point = 233°C
Molar mass = 134.24 g/mol.
Vapour pressure = 0.04 mm Hg at 20°C
Specific gravity = 0.97
Flash point = 107°C (closed cup)
Solubility:- Soluble in most Alcohols, but only slightly soluble in water
Stable. Incompatible with strong oxidizing agents, organic materials, strong bases.
Harmful if swallowed, inhaled or absorbed through the skin. Eye, skin and respiratory irritant. Eye contact may cause serious harm.
ORL-RAT LD50 980 mg per kg
IVN-MUS LD50 100 mg per kg
UN No 2810. Hazard class 6.1. Packing group III.
Harmful in the environment.
Safety glasses, adequate ventilation, avoid contact with skin or other tissue, avoid breathing fumes.
This page of data has been collected from many sources, I cannot guarantee that it is up-to-date.
Dave Cushman.
Page created 25/04/2003
Originated... 25 April 2003, Revised... 10 May 2003, Upgraded... 11 June 2006,
|