Disease Diagnosis
Bee Diseases and Afflictions
Fumidil 'B'
|
|
|
|---|
|
Disease Diagnosis Bee Diseases and Afflictions Fumidil 'B' |
Nosema Apis a Parasite of Honey Bees |
|---|
Nosema apis (Zander) is a microsporidian, it is a small, single celled parasite affecting honey bees. It causes nosemosis, mainly known by the term nosema. Although parasitic, it is often thought of and talked about as a disease. A single spore can cause infection, but the mean infective dose is generally reported to be between 20 and 90 spores per bee.
The dormant spore stage of nosema is resistant to extremes of temperature and dehydration and the spores are long lived.
The symptoms can be confused with other honey bee problems, but they are genarally...
- dysentery
- Shorter life of worker bees
- Lack of industry in the colony (slowness of development)
- Lower than normal propensity to sting
- Crawling bees
- Disjointed wings
- Distended abdomens
- High propensity to supersede
I have included disjointed wings, because many other sources quote this symptom, but I have not recognised this feature myself, maybe the effect is less severe than the 'K' wing associated with Acarine infestation.
Bees crawling in front of the hive unable to fly may be infected by nosema but there are other possible causes of this. Crawling bees, trembling wings and dislocated wings are associated with Chronic Bee Paralysis Virus (CBPV).
Spores need be ingested by a bee for the infection to be effected. Spores germinate quickly after entering the ventriculus, and the epithelial cells of the ventriculus are infected when the vegetative stage is introduced by way of the extended tubular polar filament allowing inoculation of the sporoplasm into the host cell. Once inside a cell, the vegetative stage increases in size and multiplies, effecting an apparent reduction of RNA synthesis in the host cell. In 6-10 days the infected host epithelial cell becomes filled with new spores. Epithelial cells are shed into the ventriculus where they burst - normally releasing digestive enzymes. The infected cells are shed similarly, but they release 30-50 million infective spores when they burst. With such a rapid amplification of spores, infection is rapidly spread.
Queens may become infected from various sources after they emerge from the queen cell. When the disease is severe, colony populations may become depleted and eventually reduce to a handful of bees and a queen. This is often known as "spring dwindling". Colonies that are only mildly affected can recover.
Nosema spores spread easily to other bees by spores in feaces that are voided inside the hive. The disease disrupts the digestion of pollen and shortens the life of the individual bee. A greater proportion of worker bees become infected than drones or queens, likely because of natural comb cleaning activities of young bees in which drones and queens do not participate. Nosema infected bees do not attend or feed the queen to the same extent as healthy bees, which helps the queen to escape infection. When the queen does become infected her ovaries degenerate and her egg laying rate is reduced due to atrophy of the oocytes. Queens that become infected by the parasite during the brood rearing season are likely to be superseded by the bees.
Nosema infection causes earlier worker orientation and commencement of foraging, along with earlier degeneration of hypopharyngeal glands.
There is a seasonal trend to infection, low levels during summer, a small peak during
Autumn, and a slow rise of infection during the Winter. In early spring the level of infection
increases rapidly when brood rearing starts and flight is limited due to low temperatures.
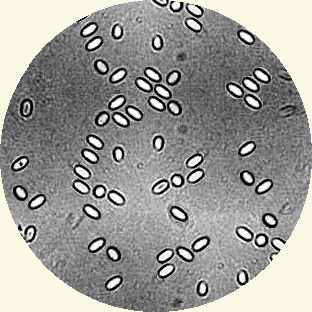
You can look for the long lived sausage shaped or rice grain shaped spores using a microscope. These spores are 4 to 6µm long and 2 to 4µm wide. They can be seen with a with a magnification between 400x to 1000x (600x prefered). Prepare your sample by macerating 10 or 15 bees under water. Use an opaque medium like Indian (Cuttlefish) ink on the slide to provide contrast.
But nosema can be detected without a microscope... Place your finger nail firmly on the sting and pull the head off exposing the mid gut. The midgut is normally tan and has a distinctive ringed appearance (similar to the segmented body of an earthworm). A midgut that is infected with nosema is off white and is a little swollen and appears slightly less segmented in appearance. This crude field test should be confirmed by microscopy if nosema is suspected to be present.
There are two schools of thought on this... My personal preference is not to treat and thus lose the susceptible bees from my collection of stock. This helps to weed out genetic weakness and promotes bees that are capable of withstanding the conditions that they are expected to work in.
Treatment can be given using the antibiotic Fumidil 'B' which is prepared from Aspergillus fumigatus, which is the causative agent of Stone Brood. Fumidil 'B' inhibits the reproduction of spores, but the antibiotic does not kill the spores themselves. Heat treatment of infected equipment, at 49° C for 24 hours or more, can be used to kill the spores.
This medication should not be fed to colonies when there is danger of contaminating the honey crop.
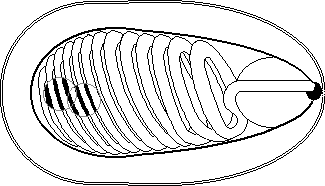
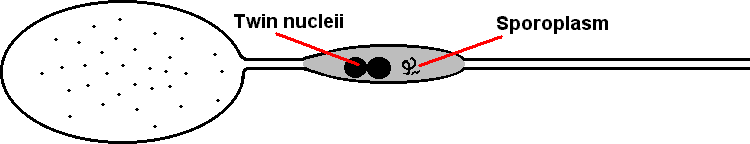
| This can be identified as several separate stages within two phases, the first of these stages is the spore itself, which has a twin nucleus as well as sporoplasm. The filament ranges from 0.1 to 0.2µm in diameter and 50 to 500µm in length. Anteriorly, the coiled polar filament passes through the polaroplast and attaches to an anchoring disc at the apex of the cell. |
|
|---|
Stage two On entering the intestine the tubule becomes extruded by eversion,
the dense glycoprotein core becomes an outer protective layer. The free end of the tube
inserts through the membrane of the host epithelial cell.
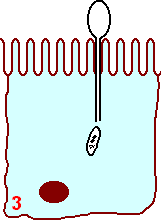
The sporoplasm is injected into the along the lumen of the polar filament into the host cell in a short time (15 to 500 msec ). This is part of merogony phase and the infectious element is known as the meront. 
Stage four (merogony)at left is the start of a progressive process of manufacturing more spores |
Inside the intestine the tubule becomes extruded and penetrates the peritrophic membrane thus entering an intestinal cell. The sporoplasm is injected into the epithelial cell along the lumen of the polar filament. 4–12 The sporoplasm (9) grows and asexually divides via quadrinucleate stages in its host cell (). Finally, (i.e. spore formation) is initiated from a diplokaryon stage (10) via a final division (). 13 When mature spores are present, host cells are disrupted and release the infectious spores into the lumen, which are voided with the feces (or infect neighboring cells). At the end of summer, development of Nosema apis may become reduced () and starts again in spring. As well as the intestine, all organs of bees become parasitized. CW, cyst wall; EN, encystation; HC, host cell; N, nucleus; NH, nucleus of host cell; PP, SP, sporoplasm; TI, tubule-injected; TU, tubule (polar filament)
Written... Summer 2000, Upgraded... 20 September 2006,
|